Pivot Bridge
Intended Use
The Pivot Bridge is a novel cardiac implant system that treats the unmet medical challenge of tricuspid regurgitation (TR). Through a simple minimally invasive and reversible procedure, Pivot Bridge intends to be used as a TR ‘pre-hab’ therapy for the period ranging from 4 days to 7 days prior to the scheduled tricuspid valve repair or tricuspid valve replacement surgery, or medical treatment.
Device
The Pivot Bridge comprises two integrated components:
Mode of Action
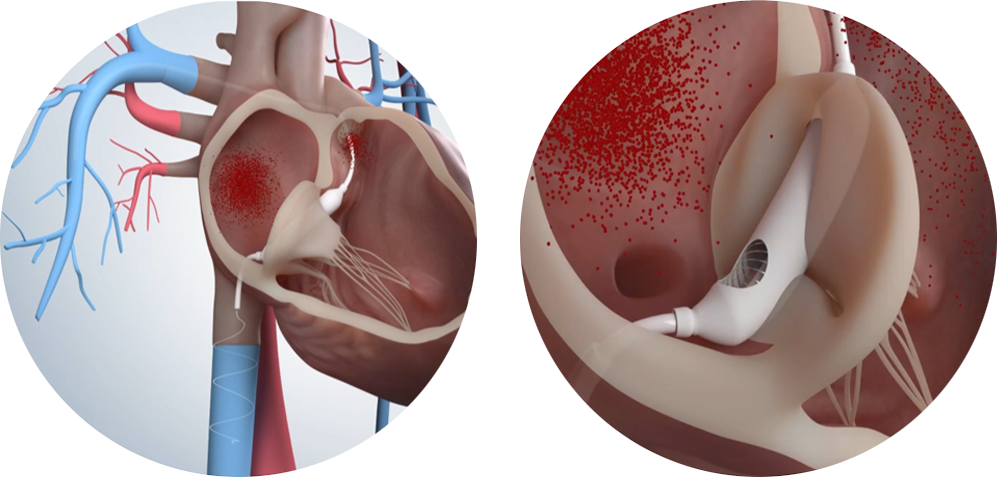
The spacer traversing the valve obliquely fills the regurgitant orifice effectively and blocks the regurgitation by enhancing the coaptation of leaflets.
Procedure
A delivery sheath containing the spacer is delivered from the puncture site in the femoral vein through the inferior vena cava to the pulmonary artery. If the sheath is uncovered, the spacer is deployed in the tricuspid valve.
The procedure is simple and fast with a short learning curve and no need for complex imaging.
Since there is no traumatic anchor, the position is adjustable, and if necessary, the device is retrievable.
Key Features
·Atraumatic anchoring structure
·Self-centering mechanism of the spacer
·Can treat TR with a large gap (torrential TR)
·Less dependent on annular or RV size
·Not dependent on IVC-RA geometry
·Reversible procedure
·Catheter retrievable if necessary (within 2-4 weeks)
Clinical outcomes and status
In the First-in-Man study of temporary (1 hour) implantation (NCT05648838), the Pivot showed TR-mitigating effect without any SAEs. The result was presented in EuroPCR 2023.
An Early Feasibility Study with the Pivot Bridge (NCT05854095) is done in 2023 and the result will be presented in TCT 2023.
Pivot Extend
Work in Progress for more Details
Hear Disease

Read our medical publications

